
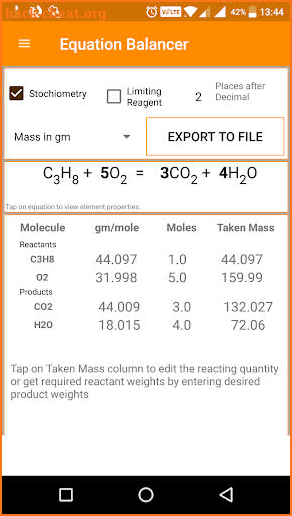
The same consistency is seen when ratios of the masses of other elements are compared. As long as we have equal numbers of hydrogen and oxygen atoms, the ratio of the masses will always be 16:1. If we have 100 atoms of each element, the ratio of the masses is approximately 1,600:100, which again reduces to 16:1. If we have 12 atoms of each element, the ratio of their total masses is approximately (12 × 16):(12 × 1), or 192:12, which also reduces to 16:1. If we have 2 atoms of each element, the ratio of their masses is approximately 32:2, which reduces to 16:1-the same ratio.
The ratio of the mass of an oxygen atom to the mass of a hydrogen atom is therefore approximately 16:1. An oxygen atom has a mass of approximately 16 u. How can we keep track of so many atoms (and molecules) at a time? We do it by using mass rather than by counting individual atoms.Ī hydrogen atom has a mass of approximately 1 u. As stated in the introduction, we deal with billions of atoms at a time. One problem we have, however, is that it is extremely difficult, if not impossible, to organize atoms one at a time. The ratio of atoms we will need to make any number of water molecules is the same: 2 hydrogen atoms to 1 oxygen atom.įigure 6.1 Water Molecules: The ratio of hydrogen atoms to oxygen atoms used to make water molecules is always 2:1, no matter how many water molecules are being made. If we want to make 5 molecules of water, we need 10 hydrogen atoms and 5 oxygen atoms. If we want to make 2 water molecules, we will need 4 hydrogen atoms and 2 oxygen atoms. In doing so, we will increase our understanding of stoichiometry, which is the study of the numerical relationships between the reactants and the products in a balanced chemical reaction.įigure 6.1 “Water Molecules” shows that we need 2 hydrogen atoms and 1 oxygen atom to make 1 water molecule. How do we compare amounts of substances to each other in chemical terms when it is so difficult to count to a hundred billion billion?Īctually, there are ways to do this, which we will explore in this chapter. Even a tiny sample of a substance will contain millions, billions, or a hundred billion billions of atoms and molecules. Although this works, most of the reactions occurring around us involve much larger amounts of chemicals. So far, we have talked about chemical reactions in terms of individual atoms and molecules. 6.1: Chapter Introduction 6.2: The Mole 6.3: Atomic and Molar Mass 6.4: Mole-Mass Conversions 6.5: Mole-Mole Relationships in Chemical Reactions 6.6: Mole-Mass and Mass-Mass Problems 6.7: Chapter Summary 6.8: References This text is published under creative commons licensing, for referencing and adaptation, please click here. Chapter 6 – Quantities in Chemical Reactions


 0 kommentar(er)
0 kommentar(er)
